Cell therapy refers to the use of biotechnology methods to obtain cells with specific functions, which are then enhanced through processes such as ex vivo expansion and specialized cultivation. These cells are endowed with augmented immune responses, pathogen and tumor cell killing capabilities, and other functions to achieve the therapeutic goals for certain diseases. Gene therapy refers to the therapeutic approach of modifying individual gene expression or repairing defective genes through methods such as gene addition, gene modification, and gene silencing. The ultimate aim is to cure diseases by rectifying abnormal genes.
|
Product Types |
Description |
Number of Approved Drugs Worldwide |
|
Gene Therapy Vector Products |
These products utilize gene therapy vectors to deliver therapeutic genes to specific cells in the patient's tissues, aiming for the expression or regulation of therapeutic proteins. This category relies on the delivery of gene therapy vectors. |
2 |
|
Cellular Products |
Using integrating viral vectors (such as lentiviral vectors), genes are introduced into precursor cells or stem cell genomes under ex vivo conditions. As cells divide, the genes are passed to descendant cells. The modified cells are then infused back into the patient. Cellular products mainly include categories such as T cells, NK cells, and stem cells. |
8 |
|
Oncolytic Virus Products |
Derived from modified oncolytic viruses with tumor-killing capabilities, these products work on the principle of utilizing the virus's specific recognition of tumor cells and the immune activation triggered upon infecting tumor cells. This leads to targeted killing of tumor cells. |
1 |
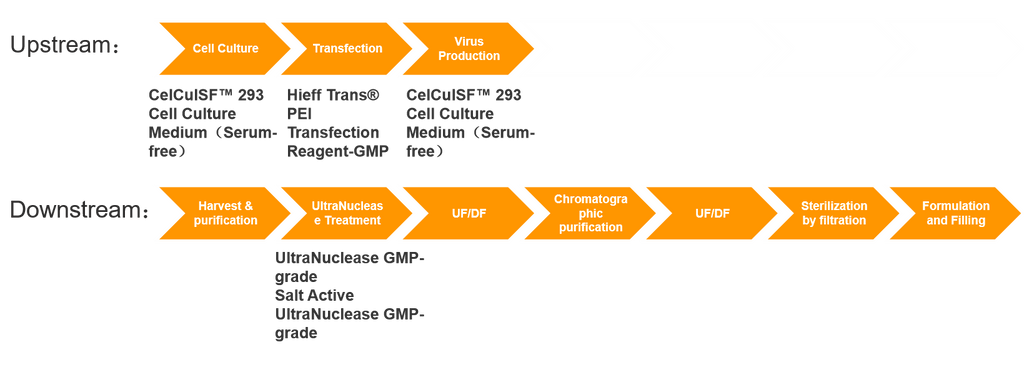
Viral Vector Production and Expression Solution
The core of Cell and Gene Therapy (CGT) drug production lies in the expression of viral vectors, a process that typically involves both upstream vector expression and downstream purification. Vector production involves highly complex processes, presents significant challenges, and often has long preparation cycles. Consequently, the global GMP production capacity for viral vectors is approaching a bottleneck, posing a major obstacle to the entire gene therapy industry's development. The development, scale-up, and GMP production of gene therapy vectors involve intricate production systems and stringent quality control systems. These encompass processes such as strain, cell, and virus banks establishment, large-scale E. coli fermentation, cell culture processes, virus harvest and purification processes, aseptic processing, and formulation and filling processes.
 AAV Production Costs
AAV Production Costs
Apart from fixed asset investments (such as equipment, cleanrooms, etc.) and labor costs, the main expenses arise from material consumption in the upstream and downstream processes. Upstream costs mainly involve raw materials like plasmids, culture media, transfection reagents, and nucleases; downstream costs mainly include chromatography resins, quality control expenses, and more.
Cationic Polymer Carrier
Cationic polymers (polymers) include polyethylenimine (PEI), poly(beta-amino ester) (PBAE), chitosan, polyacrylamide (PAH), diethylaminoethyl dextran (DEAE-dextran), poly(amidoamine) dendrimers (PAMAM), and others. The common principle of cationic polymer transfection involves complexing DNA under physiological pH conditions to prevent degradation by DNase. Subsequently, these complexes attach to the cell membrane and are taken up by endocytosis, followed by rupture and release of DNA into the cytoplasm to exert their intended functions. The primary distinction between cationic polymers and cationic lipids lies in the absence of hydrophobic portions in cationic polymers, making them completely water-soluble and allowing for convenient chemical modifications.
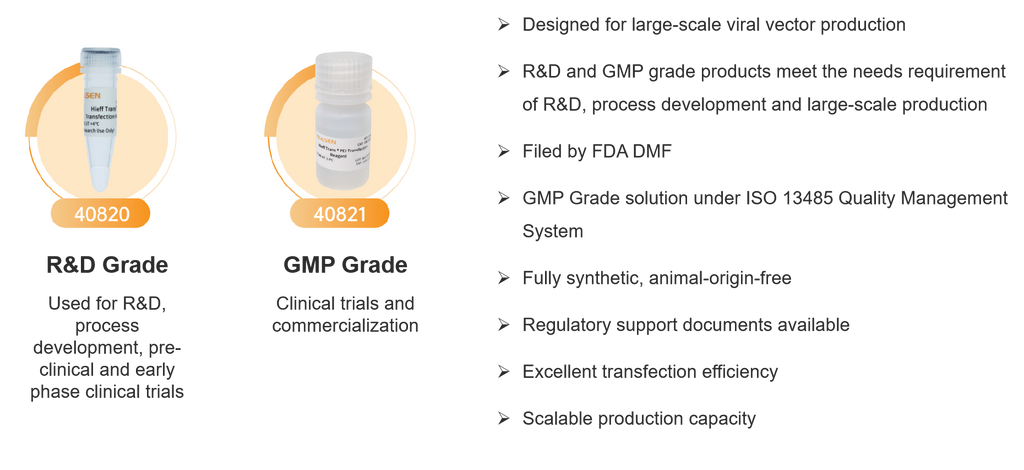
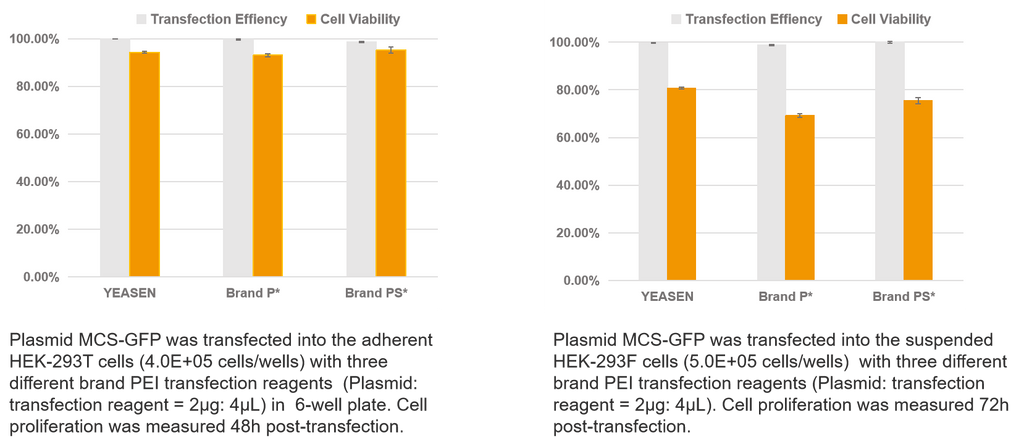
Hieff Trans® PEI Transfection Reagents
| Test Item | Standard |
R&D Grade (40820) |
GMP Grade (40821) |
| Appearance | Complete, accurate information, no damage, etc | √ | √ |
| Transfection efficiency | > 70% Transfection efficiency> 80% Cell viability | √ | √ |
| Endotoxin | < 0.5 EU/mL | √ | √ |
| Sterility | Aseptic growth | √ | √ |
| Mycoplasma Residue | Negative | √ | √ |
| pH | < 7.0 | √ | |
| Osmotic Pressure | ≤ 30 mOsm/kg | √ | |
| Impurity Residue | Methanol(≤ 0.3%) Ethyl ether(≤ 0.5%) Methylbenzene(≤ 0.002%) | √ | |
| Heavy Metal Residue | ≤ 10 ppm |
|
√ |
Transfection of Single Plasmid
Virus Titer Data
1. LV Production

2. AAV2 Production
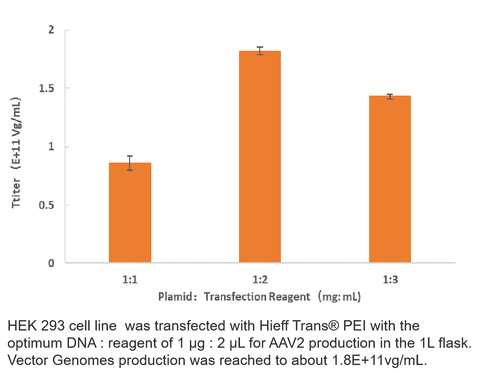
3. AAV5 Production
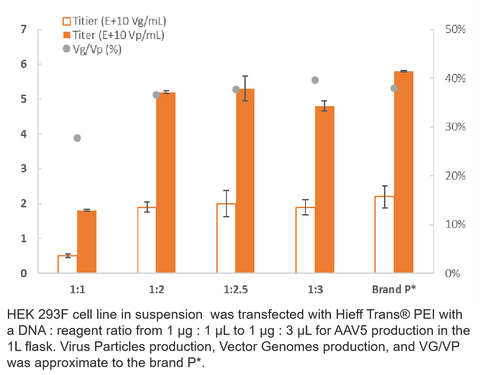
4. RV Production
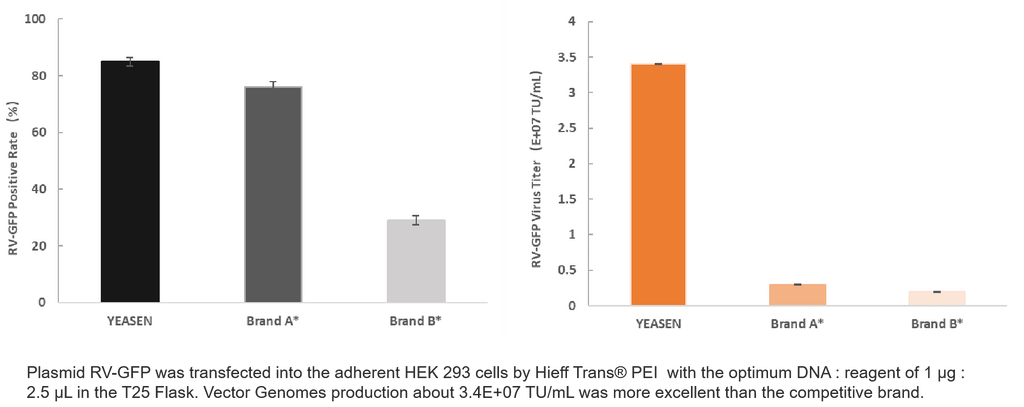
Product Information
| Product Name | Cat No. |
| E.coli Host Cell DNA Residue Detection Kit | 41308ES |
| E.coli Host Cell RNA Residue Detection Kit | 41318ES |
| E.coli HCP ELISA kit | 36712ES |
| HEK293 HCP ELISA kit | 36713ES |
| HEK293 Host Cell DNA Residue Detection Kit | 41302ES |
| HEK293 Host Cell Residue DNA Size Analysis Kit | 41316ES |
| MycAway™ Mycoplasma Real-time qPCR Detection Kit | 40618ES |
| Hieff Trans® PEI Transfection Reagent-GMP | 40821ES |
| UCF.ME® UltraNuclease GMP-grade | 20157ES |
| Salt Active UltraNuclease GMP-grade | 20159ES |
| UltraNuclease ELISA Kit | 36701ES |
| Salt Active UltraNuclease ELISA Kit | 36703ES |
| RCA (E1A) Copy number Detection Kit | 41321ES |
| Replication-competent Lentivirus (RCL) Detection Kit | 41311ES |
| MolPure™ Magnetic Residual DNA Sample Preparation Kit | 18461ES |

