In Vitro Cell Transfection Reagent Product Selection Guide
Cell transfection reagents have become routine reagents for studying and controlling gene function in eukaryotic cells. Transfection reagents are widely used in gene function research, gene expression regulation, and mutation analysis, as well as gene therapy, cell therapy, protein production, and vaccine production. So what is transfection? And how to choose a kind of transfection reagent based on your experiments?
What are the types of transfection?
The features of transfection reagents from Yeasen
How to choose a kind of transfection reagent based on your experiments?
Application case
Reference for transfection conditions
FAQs
Regard Reading
What are the types of transfection?
According to whether the nucleic acid is integrated into the host cell chromosome after transfection, it is divided into "transient" (transient transfection) and "stable" (stable transfection). The transfection efficiency, cytotoxicity, effects on normal physiology, and gene expression levels of different transfection methods are different. The principles, applications, and characteristics are compared in the following table:
Table 1 Comparison of different transfection methods
|
Technology |
Principles |
Advantages |
Disadvantages |
|
Chemical transfection method |
|||
|
Cationic liposomes |
Positively charged liposomes form complexes with negatively charged phosphate groups of nucleic acids and are endocytosed by cells. |
|
|
|
Calcium Phosphate Coprecipitation |
Calcium phosphate DNA complexes adsorb to cell membranes and are endocytosed by cells |
|
|
|
Dextran |
The complex formed by the interaction of the positively charged DEAE-dextran and the negatively charged phosphate backbone of the nucleic acid is endocytosed by the cell. |
|
|
|
Other cationic polymers |
The positively charged polymer forms a positively charged complex with the negatively charged phosphate group of the nucleic acid, then interacts with the negatively charged proteoglycan on the cell surface, and enters the cell through endocytosis. |
|
|
|
Biotransfection method |
|||
|
Viral transfection |
Instinct infects cells and delivers genetic material |
|
|
|
Physical transfection method |
|||
|
Electric transfer |
The high pulse voltage disrupts the cell membrane potential, and the DNA is introduced through the pores formed in the membrane. |
|
|
|
Biotransmission Particle Delivery (Particle Bombardment) |
The DNA is precipitated with microscopic heavy metal particles, and then the coated particles are projected into the cells with a ballistic device, and the DNA is gradually released and expressed in the cells. |
|
|
|
Microinjection |
Micromanipulation is used to inject DNA directly into the nucleus of the target cell. |
|
|
The features of transfection reagents from Yeasen
For DNA transfection reagents and RNA transfection reagents, Yeasen Biotechnology has a strong R&D and production team, continuously optimizes formulas, improves production processes, and has launched a variety of products based on cationic liposomes and cationic polymers. Scientific research institutions and enterprises provide a full range of products, and the product line covers all fields involved in transfection reagents.
|
Hieff Trans™ Suspension Cell-Free Liposomal Transfection Reagent |
|
|
40802ES |
40805ES |
|
40806ES |
40816ES |
- High efficiency: suitable for transient transfection or stable transfection of cell lines.
- Low toxicity: Transfected cells remain well-viable.
- Wide adaptability: comprehensive coverage of common cells and difficult-to-transfect primary cells.
- Easy to operate: suitable for medium in the presence of serum, without changing the medium before and after transfection.
- Cost-effective: economical and practical, high transfection efficiency, low price.
How to choose a kind of transfection reagent based on your experiments?
The selection of transfection reagents needs to be selected according to the different experimental purposes and experimental contents, such as the transfected substances, specific cells, convenience of operation and other factors.
|
Product |
Hieff Trans™ Suspension Cell-Free Liposomal Transfection Reagent |
|||
|
Cell type |
conventional cell |
conventional cell |
conventional cell |
conventional cell |
|
/ |
/ |
difficult-to-transfect cells |
difficult-to-transfect cells |
|
|
Nucleic acid type |
DNA |
DNA |
/ |
DNA |
|
siRNA |
siRNA |
siRNA |
/ |
|
|
/ |
/ |
miRNA |
/ |
|
|
/ |
/ |
mimic miRNA |
/ |
|
|
/ |
/ |
antimiRNA |
/ |
|
|
DNA/siRNA co-transfection |
DNA/siRNA co-transfection |
/ |
/ |
|
|
virus packaging |
virus packaging |
/ |
virus packaging |
Application case
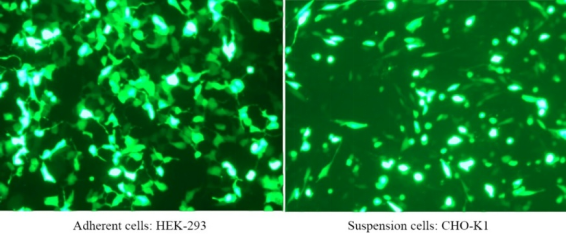
Hieff Trans™ Liposomal Transfection Reagent
Hieff Trans™ is supplied in sterile liquid form. Generally, for 24-well plate transfection, about 1.5 μL each time, 1 mL of Hieff Trans™ can do about 660 transfections; for 6-well plate, about 6 μL each time, 1 mL of Hieff Trans™ can do about 660 transfections. 160 transfections;
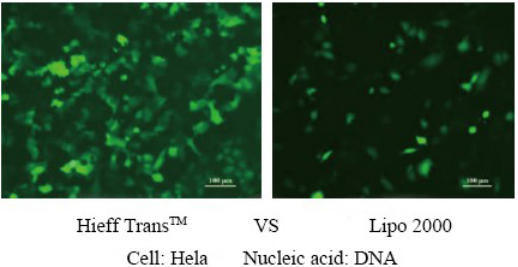
For more details, please see Confidence in transfection with Hieff Trans™ Lipofectamine Reagent
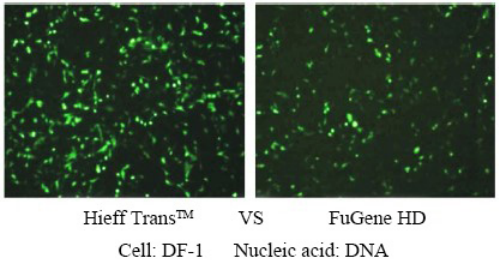
Polyethylenimine Linear (PEI) MW40000 (rapid lysis)
PEI 40000 is a highly charged cationic polymer with a molecular weight of 40,000 that binds negatively charged nucleic acid molecules very easily, forming a complex and allowing the complex to enter cells. PEI 40000 is a transient transfection reagent with low cytotoxicity, high transfection efficiency, and high gene expression efficiency in cells such as HEK293 and CHO. Linear PEI transfection reagents have been validated for a wide range of cell lines including HEK-293, HEK293T, CHO-K1, COS-1, COS-7, NIH/3T3, Sf9, HepG2, and Hela cells. The transfection efficiency is as high as 80%~90%.
For more details, please see New favorite for transfection —— Linear PEI MW 40000, a more efficient transfection reagent
Hieff Trans™ in vitro siRNA/miRNA Transfection Reagent
This product can achieve over 90% expression efficiency of 1 nM siRNA in a wide range of cell lines, avoiding off-target effects. Suitable for transfection of a variety of cells, including Hela, MCF-7, HepG2, CHO and other adherent cells; and difficult-to-transfect suspension cell lines, such as K562 or THP-1 cells, can achieve 80% silencing efficiency; Including some primary cells, primary human fibroblasts and primary human hepatocytes, etc., the silencing efficiency of 80% can be achieved.
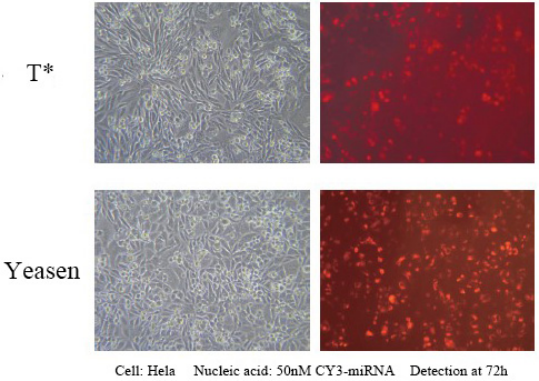
Reference for transfection conditions
In addition to the instructions for each product, customers operate according to their specific experimental content, and there will be different differences in the amount of use. According to the in vitro cell transfection conditions reported by customers after using the product, they have been sorted out for your reference.
|
Product Name/Item Number |
|||||
|
Cell |
Culture vessel |
Cell plating densities |
DNA |
Hieff trans |
Transfection efficiencies |
|
A549 |
6 well |
90% |
0.7 μg |
1.15 μL |
+++ |
|
BV 2 |
24 well |
95% |
0.2 μg |
0.2 μL |
++ |
|
C2C12 |
24 well |
80% - 90% |
1 μg |
5 μL |
++ |
|
DF 1 |
24 well |
80% - 90% |
0.5 μg |
0.5 μL |
+++ |
|
H520 |
6 well |
80% |
1.2μg |
6 μL |
++ |
|
HaCaT |
96 well |
70% |
100 ng |
1 μL |
++ |
|
HCT116 |
6 well |
90% |
4 μg |
10 μL |
++ |
|
HEK 293 |
6 well |
95% |
2 μg |
10 μL |
80 - 90% |
|
HEK 293FT |
24 well |
85% |
1 μg |
4 μL |
90% |
|
HEK 293T |
12 well |
1×105 |
1 μg |
2 μL |
+++ |
|
HEK 293T(suspension) |
30 ml |
80% |
30 μg |
60 μL |
++ |
|
Hela |
12 well |
90% |
0.2μg |
0.6 μL |
90% |
|
Hela |
12 well |
80% |
1 μg |
3 μL |
+++ |
|
HepG2 |
12 well |
80% |
1 μg |
3 μL |
++ |
|
HUVEC |
24 well |
80% |
1 μg |
2 μL |
++ |
|
MCF10A |
10 cm dish |
60% |
5 μg |
15 μL |
++ |
|
N2A |
24 well |
70% - 80% |
300 ng |
900 μL |
+ |
|
NCI H1975 |
6 well |
80% |
4 μg |
10 μL |
+++ |
|
NIH 3T3 |
6 well |
90% |
4 μg |
10 μL |
+++ |
|
Raw 264.7 |
35 mm dish |
80% |
1 μg |
2 μL |
90% |
|
Vero |
6 well |
80% |
3 μg |
9 μL |
+++ |
|
Cell |
Culture vessel |
Cell plating densities |
siRNA |
Hieff trans |
Transfection efficiencies |
|
HK2 |
6 well |
65% |
100 pmol |
6 μL |
+++ |
FAQs
1 Hieff Trans™ Liposomal Transfection Reagent
1.1 Q: Can serum be present when preparing nucleic acid transfection reagent complexes?
A: The presence of serum will affect the formation of liposomes. It is recommended to use a serum-free medium (generally MEM medium) when preparing nucleic acid transfection reagent complexes.
1.2 Q: What should I pay attention to when using Hieff Trans™ Liposomal Nucleic Acid Transfection Reagent?
A:
1) When the cells are transfected, the cell density is preferably 80%-95%, and the specific plating density is determined according to the situation of the cells;
2) Using high-purity DNA helps to obtain higher transfection efficiency;
3) DNA and transfection reagents are required to be diluted with the serum-free medium when preparing transfection complexes;
4) Antibiotics cannot be added to the medium during transfection;
5) Reagents should be stored at 2-8°C, and care should be taken to avoid repeatedly opening the lid for a long time;
6) The DNA concentration and the number of cationic liposome reagents should be optimized for the first use to obtain the maximum transfection efficiency. The ratio of DNA to transfection reagent is generally recommended to be 1:2-1:3.
1.3 Q: Does it need to be terminated after transfection?
A: No need. Liposome complexes are stable for 6 hours. If the cell medium is not changed before transfection, in order to ensure the nutrients required for normal cell growth, it is necessary to change to a new medium after 4-6 hours. However, if the medium has been changed before transfection, it is not necessary to change the medium after liposome transfection.
1.4 Q: Can co-transfection of DNA and siRNA be performed? How's the effect?
A: Yes, when DNA and siRNA are co-transfected, the siRNA transfection efficiency will be slightly worse.
1.5 Q: Can the transfection reagent be used for the transfection of lentiviral packaging?
A: Lentiviral packaging is possible.
1.6 Q: Can suspension cells be transfected with Hieff Trans™ Liposomal Nucleic Acid Transfection Reagent?
A: Hieff Trans™ Liposome Nucleic Acid Transfection Reagent can be used for suspension cell transfection, see Protocol for details. In addition, we also introduced a transfection reagent specifically for suspension cells (Cat No. 40805, Hieff Trans™ Suspension Cell-Free Liposomal Transfection Reagent)
2 Hieff Trans™ in vitro siRNA/miRNA Transfection Reagent
2.1 Q: Does the transfection reagent need to be changed after transfection?
A: This problem can be divided into two cases: 1. If there is no medium change before transfection, the medium should be changed about 6 hours after transfection to ensure the nutrients required for cell growth; 2. If there is a medium change before transfection , can be operated according to the normal operation of cultured cells? ? After the liquid change operation?
2.2 Q: Can transfection reagents be frozen?
A: It cannot be frozen, because the transfection reagent is a PEI cationic transfection reagent. Freezing at low temperatures will destroy the activity of the PEI transfection reagent. Therefore, it is best to store it at 2-8 °C to maintain the best transfection. efficacy.
Product information
| Product name | SKU | Specifications |
| Hieff Trans™ Liposomal Transfection Reagent | 40802ES02 | 0.5 mL |
| 40802ES03 | 1.0 mL | |
| 40802ES08 | 5×1mL | |
| Hieff Trans™ Suspension Cell-Free Liposomal Transfection Reagent (Inquire) | 40805ES02 | 0.5 mL |
| 40805ES03 | 1.0 mL | |
| 40805ES08 | 5×1 mL | |
| Hieff Trans™ in vitro siRNA/miRNA Transfection Reagent (Inquire) | 40806ES02 | 0.5 mL |
| 40806ES03 | 1.0 mL | |
| Polyethylenimine Linear(PEI) MW40000(rapid lysis) | 40816ES02 | 100 mg |
| 40816ES03 | 1 g | |
| 40816ES08 | 5×1 g |
Some of the articles published using our products
[1] Liu R, Yang J, et al. Optogenetic control of RNA function and metabolism using engineered light-switchable RNA-binding proteins. Nat Biotechnol. 2022 Jan 3. (IF:55)
[2] Luo J, Yang Q, et al. TFPI is a colonic crypt receptor for TcdB from hypervirulent clade 2 C. difficile. Cell. 2022 Mar 17.(IF:41.582)
[3] Zhou J, Chen P, et al. Cas12a variants designed for lower genome-wide off-target effect through stringent PAM recognition. Mol Ther. 2022 Jan 5.(IF:11.454)
[4] Chen S, Cao X, et al. circVAMP3 Drives CAPRIN1 Phase Separation and Inhibits Hepatocellular Carcinoma by Suppressing c-Myc Translation. Adv Sci (Weinh). 2022 Jan 24.(IF:16.808)
[5] Gu C, Wang Y, et al. AHSA1 is a promising therapeutic target for cellular proliferation and proteasome inhibitor resistance in multiple myeloma. J Exp Clin Cancer Res. 2022 Jan 6.(IF:11.161)
[6] Zhang Y, Yu X, et al. Splicing factor arginine/serine-rich 8 promotes multiple myeloma malignancy and bone lesion through alternative splicing of CACYBP and exosome-based cellular communication. Clin Transl Med. 2022 Feb.(IF:11.492)
[7] Qin J, Cai Y, et al. Molecular mechanism of agonism and inverse agonism in ghrelin receptor. Nat Commun. 2022 Jan 13.(IF:14.9)
[8] Tang X, Deng Z, et al. A novel protein encoded by circHNRNPU promotes multiple myeloma progression by regulating the bone marrow microenvironment and alternative splicing. J Exp Clin Cancer Res. 2022 Mar 8.(IF:11.161)
[9] Xie F, Su P, et al. Engineering Extracellular Vesicles Enriched with Palmitoylated ACE2 as COVID-19 Therapy. Adv Mater. 2021 Oct 19. (IF:30.849)
[10] Liang Y, Lu Q, et al. Reactivation of tumour suppressor in breast cancer by enhancer switching through NamiRNA network. Nucleic Acids Res. 2021 Sep 7.(IF:16.9)
[11] Fan Y, Wang J, et al. CircNR3C2 promotes HRD1-mediated tumor-suppressive effect via sponging miR-513a-3p in triple-negative breast cancer. Mol Cancer. 2021 Feb 2.(IF:27.403)
[12] Dai L, Dai Y, et al. Structural insight into BRCA1-BARD1 complex recruitment to damaged chromatin. Mol Cell. 2021 Jul 1.(IF:17.97)
[13] Zhang K, Wang A, et al. UBQLN2-HSP70 axis reduces poly-Gly-Ala aggregates and alleviates behavioral defects in the C9ORF72 animal model. Neuron. 2021 Jun 16.(IF:17.17)
[14] Li T, Chen X, et al. A synthetic BRET-based optogenetic device for pulsatile transgene expression enabling glucose homeostasis in mice. Nat Commun. 2021 Jan 27.(IF:14.92)
[15] Yan F, Huang C, et al. Threonine ADP-Ribosylation of Ubiquitin by a Bacterial Effector Family Blocks Host Ubiquitination. Mol Cell. 2020 May 21.(IF:17.97)
[16] Sun X, Peng X, et al. ADNP promotes neural differentiation by modulating Wnt/β-catenin signaling. Nat Commun. 2020 Jun 12.(IF:14.911)
[17] Yang X, Wang H, et al. Rewiring ERBB3 and ERK signaling confers resistance to FGFR1 inhibition in gastrointestinal cancer harbored an ERBB3-E928G mutation. Protein Cell. 2020 Dec.(IF:14.872)
[18] Zou Y, Wang A, et al. Analysis of redox landscapes and dynamics in living cells and in vivo using genetically encoded fluorescent sensors. Nat Protoc. 2018 Oct.(IF:13.490)
[19] Hao H, Hu S, et al. Loss of Endothelial CXCR7 Impairs Vascular Homeostasis and Cardiac Remodeling After Myocardial Infarction: Implications for Cardiovascular Drug Discovery. Circulation. 2017 Mar 28.(IF:29.69)

